Boron Based Materials And Medicinal Molecules
There has been a great deal of resurgence in boron chemistry in recent years. In the relatively small span of a decade, tricoordinatetriarylboranes and tetracoordinate BODIPY dyes have emerged as one of the most sought after candidates in various fields of “Organic Materials.”The coordinatively unsaturated tricoordinated boron is inherently Lewis acidic and forms complex with Lewis bases. This phenomenon has been well exploited in sensing small molecules like OH, H-, CN-, and F-. In biology, the active site of several protein catalases is nothing but Lewis basic hydroxyl/amine groups. Obviously a simple Lewis acid can be employed to control the activity of Lewis basic biocatalysts. It took nearly 100 years for medicinal chemists to successfully implement this simple concept in drug design. Combining our academic curiosity on boron with quest for developing new materials and medicines we are trying to find answer for fundamental questions pertaining to our day-to-day life.
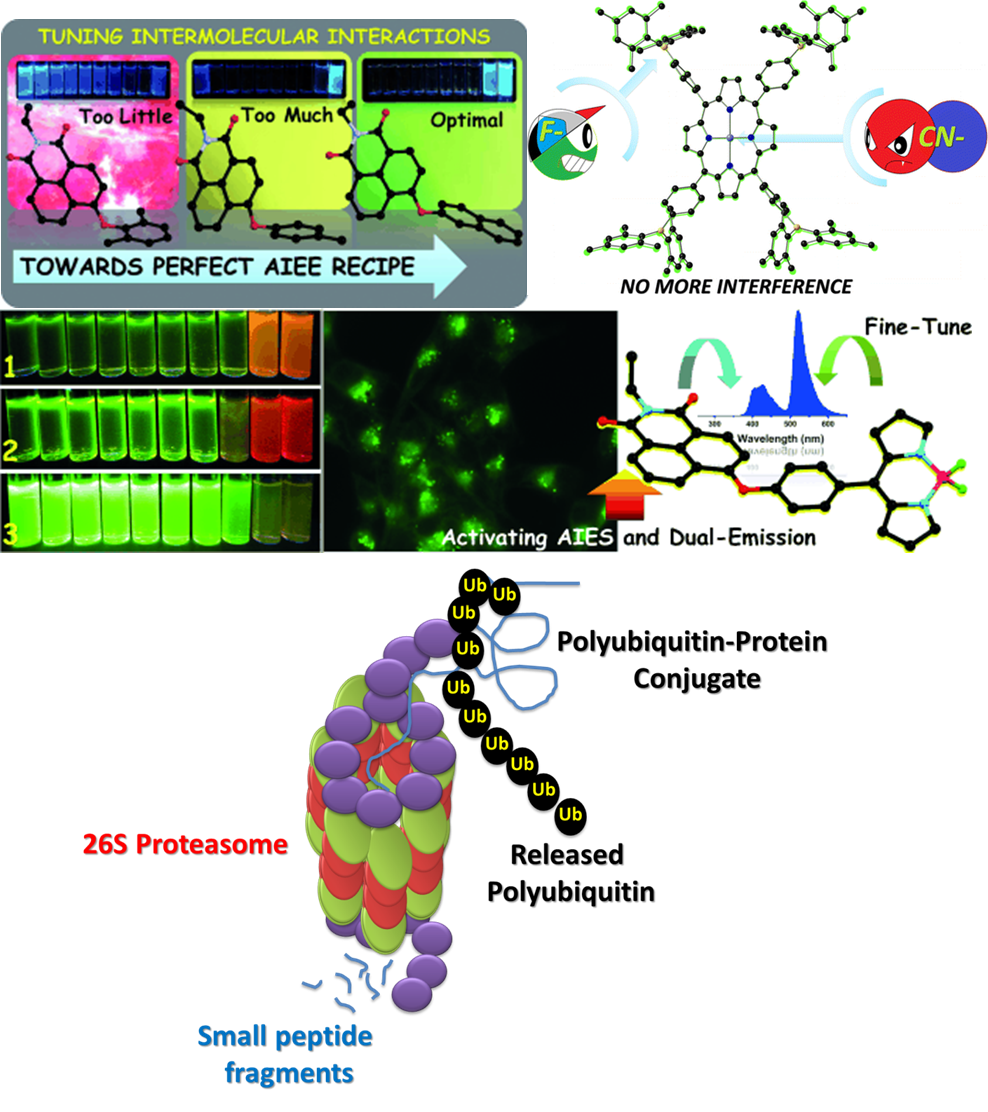
Our area of research is highly interdisciplinary, involving aspects of organic, organometallic,
polymer and Nanomaterials chemistry of BORON. Our group is engaged in the design and synthesis of new molecules/materials
containing BORON for the potential application in the field of Catalysis, Molecular Electronics (OLEDs, TFTs and solar cells),
and Chemosensory materials. We are also involved in exploiting the Lewis acidity of tricoordinated boron for the development
of boron based proteasome inhibitors (BPI) and understanding the differences in the catalytic activity of multiple proteolytic
sites in both eukaryotic and bacteria proteasomes. Our long range goals to utilise the acquired knowledge to design novel BPIs to
control proteasome activities in specific diseases.We are also actively pursuing design and development of boronopeptides and their
role as antibacterial and diabetes wound healing drug molecules.
Representative Publications
-
S. Mukherjee, P. ThilagarAngew. Chem. Int. Ed., 2019, 58, 2 – 13DOI: 10.1002/anie.201811542
-
P. Sudhakar, K. K. Neena and P. ThilagarDalton Trans., 2019, 48, 7218-7226DOI: 10.1039/C8DT04005B
An invited article for the themed issue on New Talent: Asia Pacific. -
G. Rajendra Kumar, S. Kumar Behera and P. ThilagarDalton Trans., 2019, 48, 6817DOI: 10.1039/C9DT00590K
An invited article for the themed issue on Inorganic Chemistry of the p-block Elements. -
K. K. Neena, P. Sudhakar and P. ThilagarAngew. Chem. Int. Ed., 2018, 57, 16806-16810DOI: 10.1002/anie.201811353
-
P. Sudhakar, K. K. Neena and P. ThilagarLangmuir, 2018, 34, 8170-8177DOI: 10.1021/acs.langmuir.8b01036
-
P. Sudhakar, K. K. Neena and P. ThilagarOrganometallics, 2018, 37, 1900-1909DOI: 10.1021/acs.organomet.8b00166
-
P. Sudhakar, K. K. Neena and P. ThilagarJ. Mater. Chem. C., 2017, 5, 6537-6546DOI: 10.1039/C7TC01676J
-
K. K. Neena and P .ThilagarOrganometallics, 2017, 36, 2692-2701DOI: 10.1021/acs.organomet.7b00332
An invited article for the special issue on Tailoring the opto-electronic properties of organometallic compounds with main group elements. -
K. K. Neena, P. Sudhakar, K. Dipak and P. ThilagarChem Commun, 2017, 53, 3641-3644DOI: 10.1039/C6CC09717K
-
S.Sarkar, G. Rajendrakumar and P ThilagarChem. Commun., 2016, 52, 4175 – 4178DOI: 10.1039/C6CC00823B
-
G. Rajendrakumar, S. K. Sarkar and P. ThilagarPhys. Chem. Chem. Phys., 2015, 17, 30424 – 30432DOI:: 10.1039/C5CP05378A
-
A. Swamy ,Sanjoy Mukherjee, P. ThilagarAnal. Chem., 2014, 86, 3616-3624DOI: 10.1021/ac500230p
-
Sanjoy Mukherjee and P. ThilagarChem. Eur. J.,2014, 20, 8012-8023DOI: 10.1002/chem.201304694
-
C. A. Swamy and P. ThilagarInorg. Chem., 2014, 53, 2776-2786DOI: 10.1021/ic402898n
-
C. A. Swamy ,Sanjoy Mukherjee and P. ThilagarChem. Commun., 2013, 49, 993-995DOI: 10.1039/c2cc38352g
-
Chinna Ayya Swamy P and Sanjoy Mukherjee and Pakkirisamy ThilagarJournal of Materials Chemistry C (2013), 1, 31, 4691DOI: 10.1039/C3TC30632A

