Spectroscopy, Structure and Bonding, Kinetics and Dynamics
Prof. Arunan and Dr. Murthy S. Gudipati, JPL, NASA will be giving a course on Astrochemistry in January 2026 semester. The schedule and venue are given here.
Prof. Arunan has been listed as one of the 2024 PCCP (Physical Chemistry Chemical Physics) outstanding peer reviewers.
Note: UG/PG students from outside IISc - Internship/summmer projects not available in our laboratory.
Click here to visit our group home page.
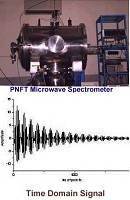
We have built the first Pulsed Nozzle Fourier Transform Microwave Spectrometer in India. This is being used to study the rotational spectra of weakly bound complexes formed in a supersonic expansion. Based on the bulk properties, Pauling had concluded that water being a liquid can form hydrogen bonds and hydrogen sulphide remains a gas as it has weak van der Waals interactions. After a detailed investigations of a series of complexes formed by H2O and H2S, we showed that both can form hydrogen bonds. Following our efforts, IUPAC changed the definition of hydrogen bond in 2011. Microwave spectroscopic and theoretical investigations on argon-proparhyl alcohol led us to the 'carbon bonding', analogous to the hydrogen bonding. Our work on 'carbon bonding' has in a way completed the quest of chemists to find intermolecular bonding by atoms beyond hydrogen.
Shock tube studies are being carried out, in collaboration with Aerospace Engineering Department,
on the kinetics of unimolecular and bimolecular chemical reactions. A single pulse shock tube facility has been
used to study thermal decomposition of several molecules. This facility has also been used to measure the ignition
delay of fuels that are of interest to Space and Defense organizations. For more information,
please visit the website of the
Laboratory for Hypersonic and Shockwave Research (LHSR).
Representative Publications
-
S Gupta, C. N. Cummings, N. R. Walker and E. ArunanJ. Mol. Spectrosc. 407 (2025) 111968.DOI: 10.1016/j.jms.2024.111968
-
E. Arunan, P. Metrangolo, G. Resnati, and S. ScheinerCryst. Growth Des. (2024) 24, 8153-8158.DOI: 10.1021/acs.cgd.4c00982
-
S. Gupta, C. N. Cummings, N. R. Walker and E. ArunanPhys. Chem. Chem. Phys., (2024),26, 19795-19811
-
S. Chakraborty, S. N. Yurchenko, R. Georges, A. Simon, O. Lacinbala, V. Chandrasekaran, V. Jayaram, E. Dartois, S. Kassi, A. Gusdorf, P. Lesaffre, G. Jagadeesh, E. Arunan and L. BiennierAstronomy & Astrophysics, (2024), 681, A39.DOI: 10.1051/0004-6361/202347035
-
S. Hossain, M. K. Singh, J. Gopalan and E. ArunanCombustion and Flame 256 (2023) 112773.DOI: 10.1016/j.combustflame.2023.112773
-
S. Hossain, M. K. Singh, J. Gopalan and E. ArunanCombustion and Flame 256 (2023) 112776.DOI: 10.1016/j.combustflame.2023.112776
-
A. Das and E. ArunanPhys. Chem. Chem. Phys., (2023), 25, 22583.DOI: 10.1039/D3CP00370A

