Research interest
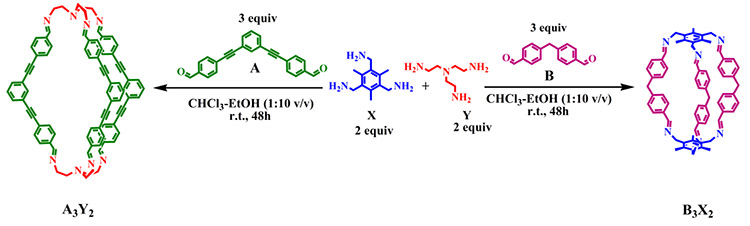
Self-sorted synthesis of nanoscopic organic cages:- Self-sorting is a well-established synthetic protocol in biological world. It is defined as a spontaneous association through mutual recognition of complementary building units into a well-defined ordered architecture, within a random reaction mixture. In order to understand such nature’s own process in a better way immense efforts have been paid off in last few decades to construct several artificial architectures utilizing non-covalent interactions. On the contrary, hitherto covalent self-sorted systems are very few in the literature. In our recent work (Mukherjee et al. J. Am. Chem. Soc., 2013, 135, 354) we are successfully able to show for the first time unprecedented self-sorting of three-dimensional nanoscopic organic cages driven by the dynamic imine bond. We have further established that such self–sorting process can be regulated by supramolecualar interaction especially by H-bonding (Mukherjee et al. Chem. Eur. J. Accepted article.)
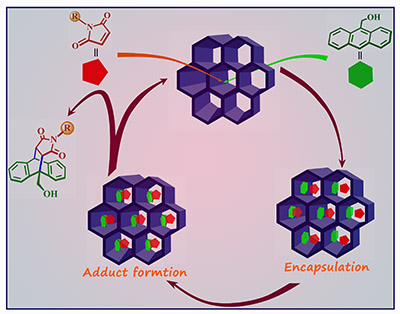
Catalysis in confined nano-space (Mimicking Nature's enzyme catalysis): Organic reaction through selective encapsulation of reactants into nano-size pores becomes one of our current interests. The many-fold enhancement in reactivity of the reaction can be attributed due to reduction of entropy loss and lowering of transition state energy that arise due to the organization of the reactants in suitable positions or its encapsulation in a confined space. Compared to homogeneous catalysis, heterogeneous catalysis is widely used for industrial applications because of easy separation and reusability, which often leads to lower production costs. In this regard, we have been using the confined nanospace of finite nanocages to perform organic transformations in aqueous medium (Chem. Eur. J. 2012, 18, 12322).Recently we have used the same concept using the nanopores of electron rich MOF as reaction vessel to perform catalytic Diels-Alder reactions under mild reaction condition (Chem. Commun., 2013, 49, 7439).
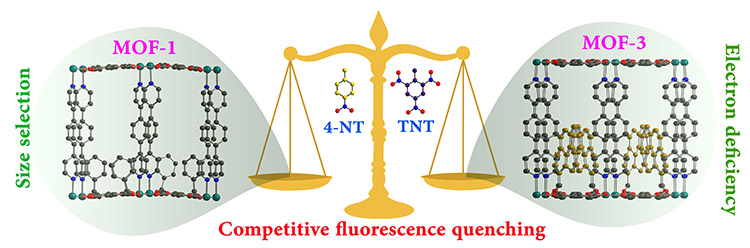
Sensing explosives: Among the several reported materials, in recent times, supramolecular architectures and extended MOFs have attracted enormous attention as a chemo-sensor. Ddetection of nitro-aromatics (NACs) has become one of growing research topics because of their uses as explosives. Apart from their explosive behavior, it has been established that many of them act as potential environment pollutants. The selective and sensitive detection mainly depends on the electron density and the ability of the sensors to donate electrons. We have established two distinct strategies to enhance the electron density of the sensors. Firstly, introducing an electron rich π conjugated fluorescent ligand, which mainly develops luminescence in the backbone of thesensors (Chem. Commun. 2011, 47, 12137). The second approach was recently established from our group introducing fluorescent tags to the same structure (Chem. Eur. J. 2014, 20, 2276).
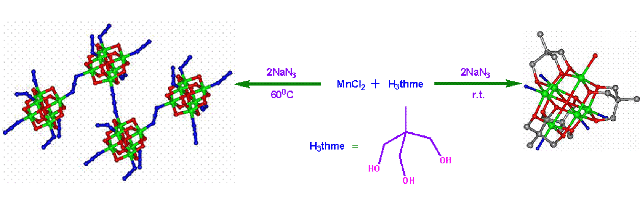
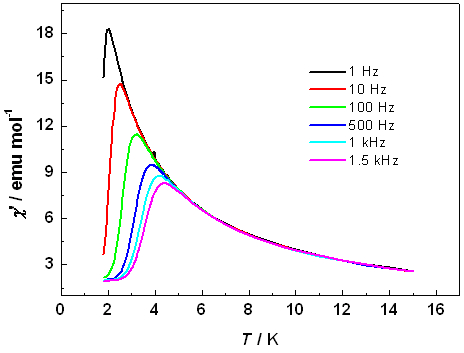
Polynuclear clusters as single molecule magnets:- One of the most pressing technology needs today is to find more efficient ways to store and process digital information. There are a couple of possibilitys: one is to aqueeze more data onto storage devices by making currently used magnetic nanoparticles even smaller. Another is to develop fundamentally different ways to process potential applications, the use of chemistry to develop new materials will be critical. That's where single-molecule magnets (SMMs) may fit in. Conventional, magnets rely on the collective behavior of the unpaired electron spins of hundrads of thousands or millions of individual metal centers in a particle or vulk material. SMMs, on the other hand, are transition-metal clusters that individual exibit the classical properties of a magnet below a critical temperature called the blocking temperature, which is currently limited to about 4 K. Our main interest on this project is to assemble smaller simple magnetic clusters by several linkers to larger arrays to see whether the resulting larger arrays are improved magnets compared to the starting small clusters. Using this methodology we have recently synthesized a couple of Mn9 nano-funnels from a simple and known antiferromagnetic Mn3 triangle using a new trihydroxy linker. Detail ac as well as dc magnetic studies revealed that the Mn9-larger clusters are new kinds of single molecule magnet. Similarly, a new m6-oxo-centered Mn6 cluster was prepared and used as the secondary building unit to prepare an azido bridged polymer of this cluster with the retention of the parent Mn6 core. In this case also the magnetic behaviour changed upon the formation of poly-cluster (K. C. Mondal, M. Drew, P. S. Mukherjee, Inorg. Chem. 2007, 46, 5625; Chem. Eur. J. 2013, ).
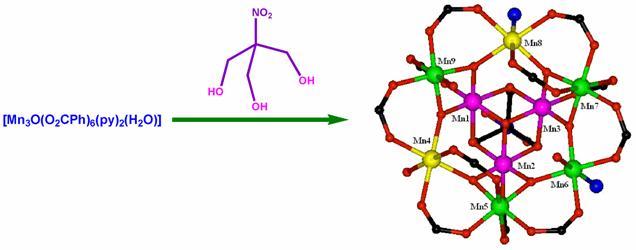
Mn-single molecule magnet
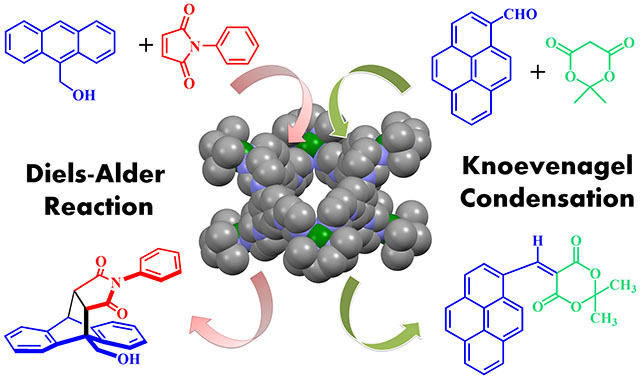
Catalysis in Self-Assembled cage: Investigations on cavity-induced entropically favorable organic transformations in “green” aqueous media are ultimately aimed at mimicking enzyme functions. In 1948, Pauling postulated that enzymes catalyze a reaction through specifically binding with the transition state. This preferential binding stabilizes the transition state, thereby lowering the activation energy barrier for that reaction, which allows for easy formation of the product. Since the 1990s, various research groups around the world have investigated the formation of nanocages with hollow structures that can encapsulate guest molecule as reaction vessels to mimic enzymatic functions. Non-covalent cages are preferred over covalent cages because guest-exchange is more eloquent and tedious multistep syntheses, which are necessary for covalent synthesis, can be avoided. Hollow architectures, which are formed by metal–ligand coordination-driven self-assembly, are particularly appealing because the designer can chose from a variety of donors and acceptors with suitable geometries and modify the organic backbone to suit the specific demands of the reaction to be studied. We have reported several new hollow water-soluble cages. The cages were successfully crystallized with hydrophobic guests to confirm the hydrophobic nature of the cavity. The hydrophobic confined nanospace of the cages was used to perform catalytic Knoevenagel condensation of a series of different aromatic aldehydes with active methylene compounds. We have also established that the cavity of the cage enhanced the rate of the Diels–Alder reaction of 9-hydroxymethylanthracene with N-phenylmaleimide or N-cyclohexylmaleimide (Chem. Eur. J. 2012, 18, 12322).