Research Interests
Current emphasis of Research is in the following areas:a) Chemistry of elemental boron, boron clusters, boranes, metallaboranes and metal borides
b) Transition metal organometallics, activation of small molecules, C-H bond activation, agostic interactions, C-C coupling reactions
C) H-bond, and weak interactions across the periodic table
d) Chemistry of heavier main group elements.
Electron Counting Rules, Elemental Boron, Boron-Rich Solids, boron-fullerenes and boron-nanotubes:
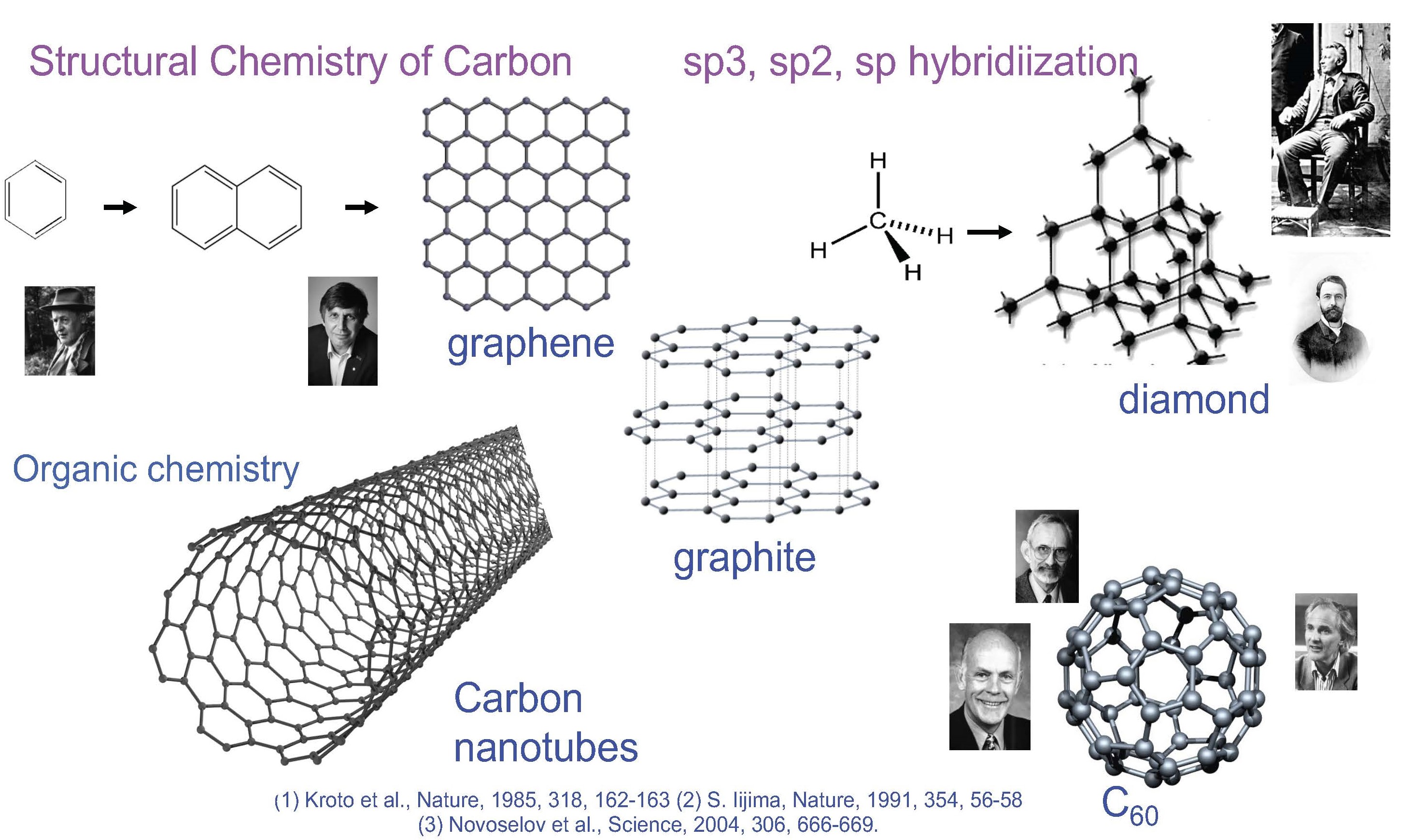
One of the first benefits of quantum mechanics to organic chemistry came in the form of π MO theory and the Hückel π electron rule. In addition to generating a large area of chemistry of the condensed aromatics, the Hückel rule brought a conceptual relationship between benzene, naphthalene and other condensed aromatics on the one hand and graphite on the other.
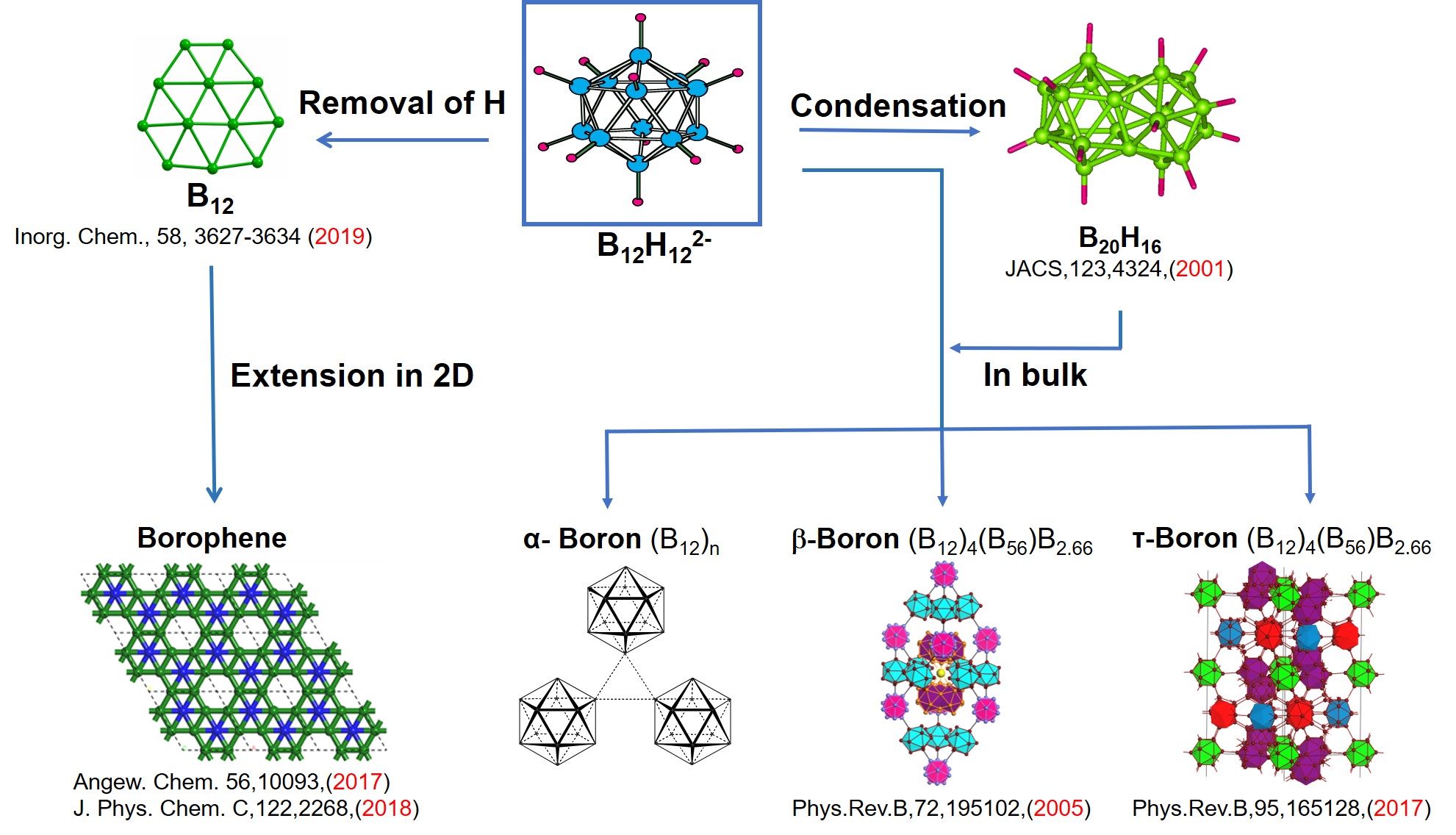
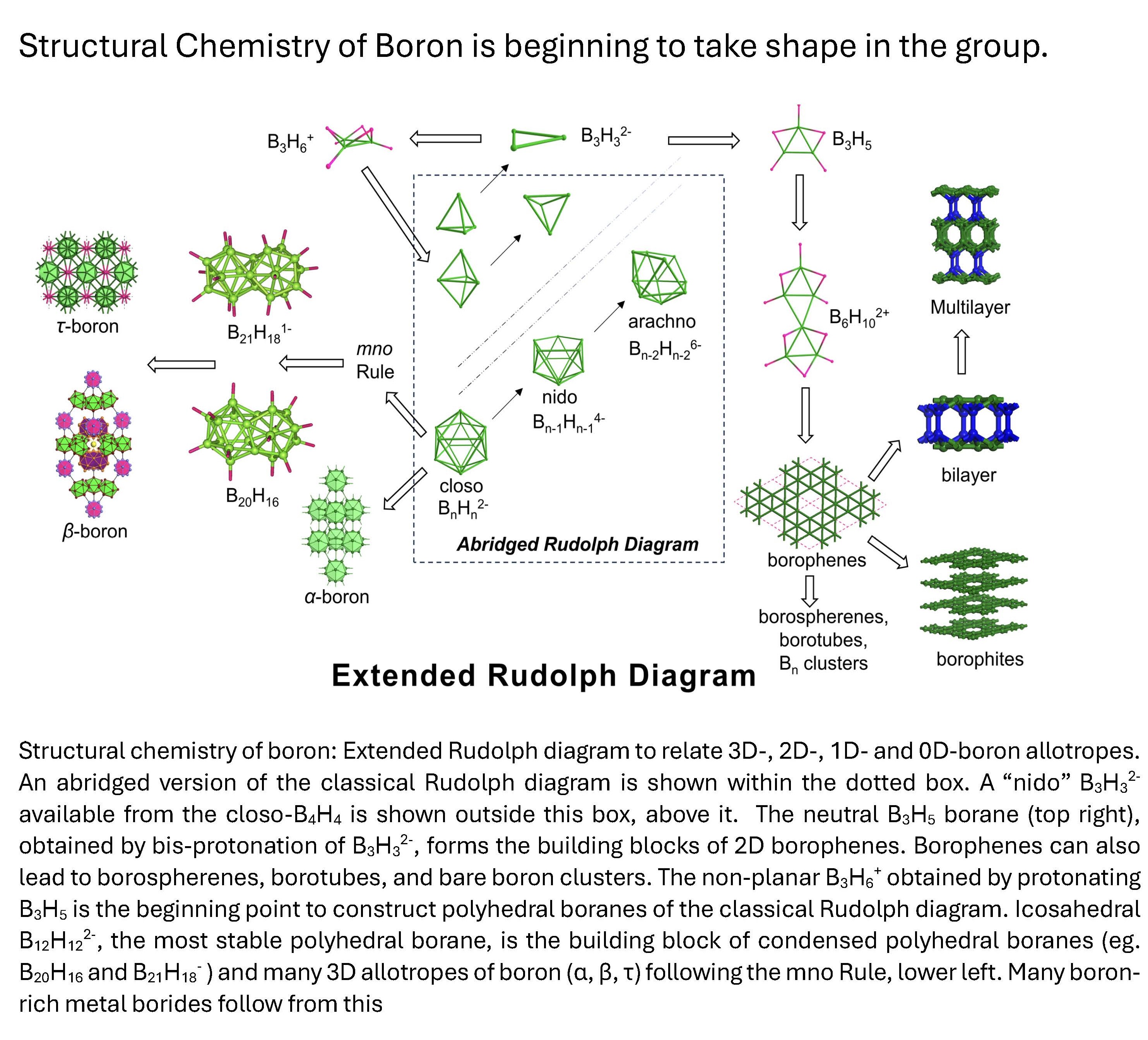
A similar understanding of the structure of boranes and the allotropes of boron is lacking. An electron counting rule to condense polyhedral boranes and its application to relate polycondensed boranes to beta-rhombohedral boron is being sought. This process helps in bringing conceptual links between carbon and boron chemistry and an understanding of the structure of boron-rich solids. This has also led to the design of boron based stuffed fullerenes and boron nanotubes. Borospherenes, tau-boron and boron-rich metal borides are amongst the current areas of research.
Jemmis group has begun the study of boron equivalents of graphenes. The inevitability of hexagonal holes in the triangular boron network of borophenes is demonstrated. The effect of metal templates and multilayers of borophenes are currently being probed. We also study the structure and reactions of smaller molecules of boron and heavier main group elements with unusual multiple bonds.
Transition Metal Organometallics:
In the Year of the Periodic Table of Elements, we propose a way to make comparisons across the periodic table for a given chemical reaction considering elements from four major blocks: main group (MG), transition metals (TM), lanthanides (Ln) and actinides (An). In general, chemists often attempt to compare the chemiStry of the lanthanides and the actinides. Is there any relation between the chemistry of MG with that of Ln or An? Is it possible to see a specific reaction which spans the four blocks of the periodic table so that more insight is obtained on the issue? We probe these general questions by focusing on a particular class of molecules, di-acetylide bridged bimetallic complexes [L]M(μ-CCR)2M[L] and their C-C coupled products [L]M(μ-RC4R)M[L], where M represents selected elements from the MG, TM, Ln and An. The approach can be extended to other reactions. Though we do not conclude that this is universally possible, this is an approach not tried before.
Although the product distributions of spin forbidden ion-molecule reactions in the gas-phase is well studied, reactions that study the dependence of product distributions as a function of spin-state in solution have been rare. A a unique isocyanide coupling reaction mediated by Cr-Cr quintuple bonded complex, to give products with different spin multiplicities gave us opportunity to study computationally the electronic structure of the products with different spin states, mechanistic pathways for their formation, and MECPs, to understand their product distributions. The approach used in this study involving multiple potential energy surfaces lends itself to obvious extensions.
Much chemistry of boron is discovered by mimicking carbon, compensating the inherent electron deficiency. Two important strategies, use of Lewis donor ligands and transition metal templates, led to stabilization of boron analogs of alkanes, alkenes, alkynes, allenes and cumulenes. In addition, there are reports for the stabilization of ethyl cation analog of boron [B2H5]-. Several [B2H5]- complexes with transition metals are known, all with non-classical [B2H5]- core. We show how transition metal template stabilizes classical [B2H5]- unit in [(Cp*Ta)2(μ,η2:η2-B2H5)(μ-H)(κ2,μ-S2CH2)2] theoretically and experimentally in collaboration with Prof. Sundargopal Ghosh's group, IITM.
Just about any transition metal is shown to help in C-H bond activation. This is remarkable for the following reason. The energy of a C-H bond remains approximately constant in most organic ligands. On the other hand the energy of the atomic orbitals of the metal from Sc to Ni varies substantially. A general understanding of the ways in which different ligand combinations work together to make any transition metal fragment to activate C-H bond and in some instances, stabilize them on the way as agostic structures, is being pursued. Similar studies are planned for other single bond activation processes. Jemmis group also collaborates with experimentalists in these areas.
The idea of isolobal connections that exist between apparently unrelated species is being studied in a variety of contexts. Double insertion reactions of benzynes to naphthalenes are used as models for similar reactions in carborynes. Analogues of olefin metathesis in the chemistry of boron is also being followed. We are currently looking at ways of stabilizing boron equivalents of Cp2Zr(C4H4) where C4H4 is replaced by B4R4.
Weak Hydrogen Bonds and other Non-covalent Interactions:
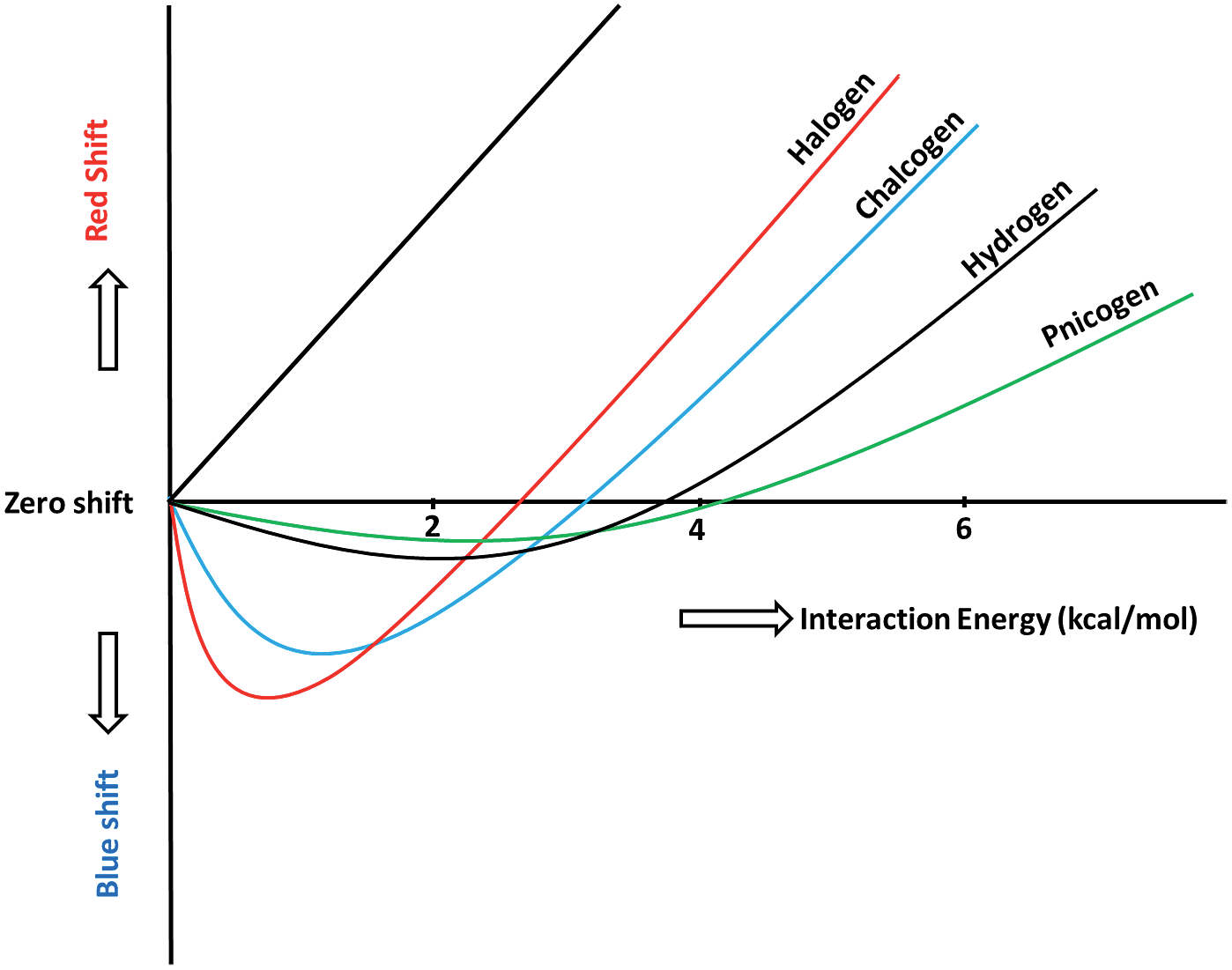
Nature of bonding in the weakest of the Weak interactions, viz. the C-H...π interactions has been a point of interest in recent years. The unexpected shortening of the C-H bond as a consequence of the C-H...π interactions has been studied by us theoretically and experimentally in collaboration with many research groups. The experimental strategy has been to isolate the system in an argon matrix and use surface FTIR spectroscopy in collaboration with experimental groups. Theoretically the spectra and the structure of these systems have been studied. Jemmis group dissected the total interaction energy into various components and now understand, for example, the reasons for the near equal interaction energy of added CHCl3 molecules to HCCH up to four CHCl3 around the HCCH. Implications of the shortening of the C-H bondlengths involved in C-H...π interaction are being pursued. Dr. Jemmis provided a general understanding for the blue-shifted H-Bonds in X-H---Y and are in the process of generalizing this to any weak bond X-Z---Y where Z is any atom in the periodic table of elements. Examples of the latter such as the Halogen-Bond, and Chalcogen Bonds are now well known. A plethora of other Z-Bonds are waiting to happen. We have started to apply the weak interactions such as the halogen bonds in novel ways, such as strengthening the sextuple bond of Cr2 and Mo2.
Novel Ideas in Structure and Bonding:
The accepted concepts of structure and bonding is tested to its limits so that these could be broken and novel arrangements of atoms which are contrary to the chemical commonsense can be designed. Six valence electron tri-coordinate pyramidal arrangements have been stabilized for boron and aluminum. A comparison of the bonding capabilities of heavier main group elements indicate that the rules of bonding that are taken as standard for the lighter elements, exemplified by carbon, is more of an exception than the rule. The general idea that bond length is proportional to bond strength and that sigma bonds are stronger than pi bonds is common in chemistry. Though difficult to see in large numbers, it is reasonable to anticipate short bond lengths if pi-only bonds can be designed. Jemmis group has recently designed aromatic hyper coordinate molecules involving P and S, such a (PF3)3-2 and (SF2H2)3 using qualitative perturbation theory using variations in electronegativity of substituents as a perturbation.
Boranes and Elemental Boron
Understanding of polyhedral molecules and their relationship to planar molecules on one hand and to covalent solids on the other has been a major research direction in the group. The concept of aromaticity that has been successfully used in two dimensional systems has been extended to three dimensionaly delocalized systems such as polyhedral boranes, carboranes and related molecules. In addition to the electron count, the compatibility of orbitals in overlap has been used to explain and to predict the relative stability of these systems. This experinece has led to the theoretical study of clusters of various sizes such as the fullerenes, stuffed fullerenes and Si coated fullerenes.
The structural relation between benzene, condensed aromatics and graphite have been a guiding principle in the chemistry of carbon. The sp2 hybridization, the Hueckel 4n+2 Rule, the sheet structure of graphite, and the spherical C60 which retains the sp2 hybridization with some strain have all become a part of the language of chemists. Similar connections amongst the tetrahedral carbon compounds with sp3 hybridization and diamond are taken for granted. It is difficult to imagine an era when these connections were not known.
Boron, the element next to carbon, also forms several polymorphs which are covalent solids. These are far more complex than those of carbon, but no less exciting. The most stable polymorph, the beta-rhombohedral boron, has 105 atoms in its idealized unit cell. The compounds of boron has defied conventional bonding characteristics that are brought over from the carbon family. The hydrocarbon equivalents are the boranes, which come in all kinds of polyhedral structures- the dianionic closo-polyhedral boranes (BnHn(-2)) and the many nido, arachno and other varieties. It would be ideal to link the monomeric polyhedral boranes to the polymorphs of elemental boron, just as polycondensation of benzene structurally speaking leads to graphite . We have found a new electron counting rule which helps to predict the electronic requirements for the condensation of polyhedral boranes. This naturally explains the beta-rhombohedral boron structure.
The first condensed polyhedral borane, B20H16, the product of the fusion of two icosahedral boranes sharing four vertices, was synthesised over 40 years ago by Lipscomb and Muetterties independently. In contrast to the icosahedral borane dianion, B20H16 is neutral. This was puzzling and no attempt was made to follow up this trail for a long time. In the first of two consecutive papers that will appear shortly in JACS we have provided an electron counting rule that is applicable to condensed polyhedral boranes, metallaboranes, metallocenes and any combination of these. According to this "mno rule", m+n+o number of skeletal electron pairs are required for stability of these condensed systems. Here m = number of vertices, n = number of polyhedra and o = the number of single-atom-bridging two polyhedra. The n+1 rule, also known as the Wades rule, is a special case of the mno rule when the number of polyhedra m =1 and the number of single-atom-bridges = 0. Thus the B20H16 requires 22 electron pairs (m=2, n= 20, o=0). The number of skeletal electron pairs is calculated as follows: 16 BH groups donate one electron pair each. The four boron atoms provide 6 electron pairs so that the sum is already 22 so that B20H16 is neutral.
In the second paper we apply the mno rule to understand, for the first time, the electronic requirements of the thermodynamically most favorable polymorph of elemental boron, the beta-rhombohedral boron. The idealised unit cell of this polymorph has 105 atoms. These can be divided into two groups of 48 and 57 atoms. The former is the equivalent of 4 B12 units obtained by breaking 2c-2e bonds. Each of these B12 units requires 2 additional electrons, reminiscent of the B12 H12(-2) ion. Thus this B48 unit is electron deficient to the tune of 8 electrons. The B57 unit which cannot be further divided into smaller fragments by breaking 2c-2e bonds has to be treated as such. According to the mno rule the B57 unit has three additional electrons than it requires. Thus the idealized B105 unit requires an additional five(8-3) electrons. However elemental boron doesnot show this electron deficiency experimentally. Nature finds an ingeneous solution by forcing the element to have "extra" boron atoms and partial occupancy simultaneously. Careful X-ray structural studies had shown that the B57 unit has always a partial occupancy corresponding to a total of one boron atom (ie three valance electrons). This takes care of the three extra electrons. On the other hand several large formally vacant holes in the B48 part has partial occupancies totalling to B2.66 which equals 8 (3x2.66=8) electrons.
These results will enrich the chemistry polycondensed polyhedral boranes and provide new tools to tune the properties of boron and boron rich solids. Several attempts along this direction are underway.
The methodolgy of relating the electronic structure of well-defined family of compounds of an element to the structure of its polymorphs is obviously amenable to extensions. Especially important is the realization that small variations in partial occupancies in a solid may not be accidental and may be necessitated by the electronic requirements.