Our Research
Main Group Synthesis, Catalysis and Small Molecules Activation
Main group elements have proven to be indispensable tools in modern chemistry, playing crucial roles in the synthesis of novel compounds, catalysis, and small molecule activation. Our group is interested in designing novel Frustrated Lewis Pairs (FLPs) based on Group 13, 14 and 15 elements to activate small molecules like CO₂, H₂, N₂, N₂O, SO₂, etc, and further convert them into value-added products under ambient conditions.We are also focused on developing a novel class of bench-stable Lewis acids for catalytic transformations.
The reversible Z-E photoisomerization of the azo group allows innumerable applications of aromatic azo compounds, including photoswitches, dyes, antibiotics and molecular machines. Our group is engaged in utilizing photoswitching behaviour of azoarenes to difunctionalize the N=N double bond to form N-X (X=P, B, Si) bonds under metal- or catalyst-free conditions.
Chemistry of Nucleophilic Boron Species
Main group Lewis acids such as boron, aluminium, tin, and silicon are known for their ability to accept electrons via low-lying empty orbitals. [cite: 9] [cite_start]Among these, boron is particularly notable for its inherent electrophilicity due to its vacant p-orbital. [cite: 10] [cite_start]Interestingly, this property can be reversed; upon coordination with Lewis bases or nucleophiles, boron can adopt a nucleophilic character, either through electron-density redistribution or by forming anionic, tetravalent borate species.
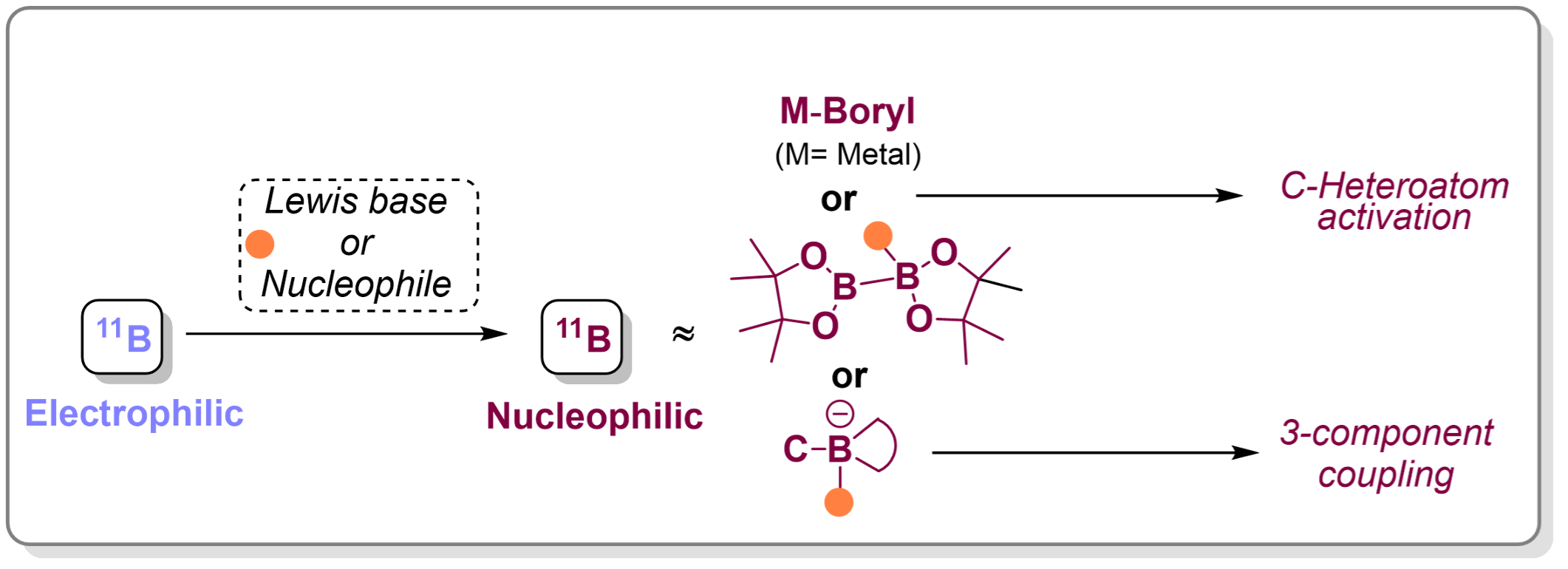
Our research group is interested in exploring this unique reactivity switch to develop both metal-catalysed and metal-free transformations.We are particularly focused on the activation of strong C–X (X = heteroatom) bonds and the facilitation of 1,2-metallate rearrangements.These strategies enable the efficient synthesis of complex, functionally rich molecules under mild conditions, with applications in multicomponent reactions and the construction of bioactive boron-containing compounds.
Lewis Acid Main Group Chemistry
Boron Assisted Tri-Fluoromethylation Reaction:
Incorporating a trifluoromethyl group to a molecule increases its electronegativity, lipophilicity, and metabolic stability, making it highly useful in pharmaceuticals and agrochemicals.We are concentrating on boron-assisted simultaneous formation of C-CF₃ and C-X (C/heteroatom) bonds on carbon-carbon olefinic bonds in a regioselective manner, without the use of transition metals or additives.
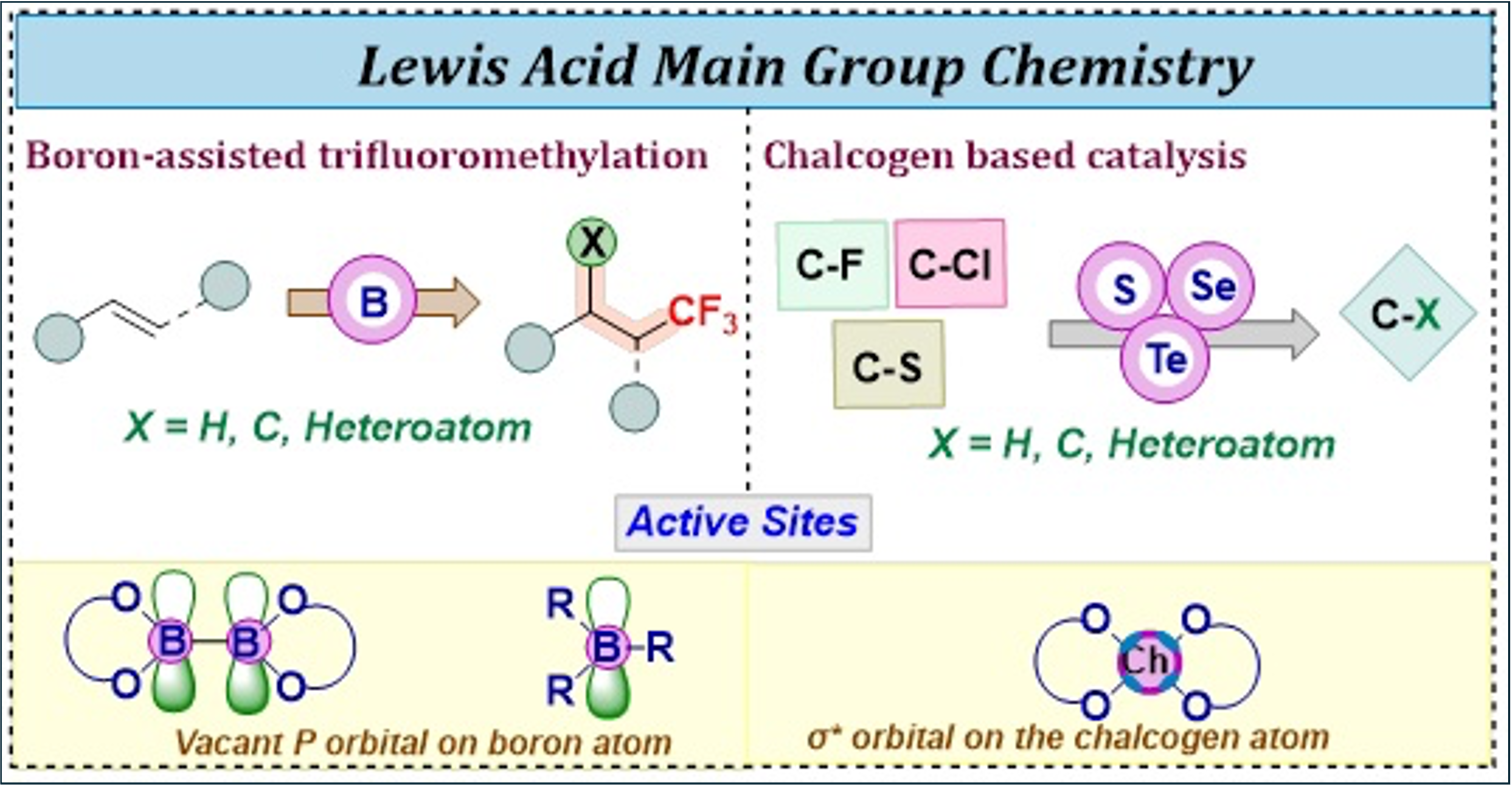
Chalcogen-Based Lewis acids Catalysts:
In the area of Main-Group chemistry, chalcogens are increasingly studied for their potential as Lewis acids in various applications.Our research focuses on developing chalcogen-based Lewis acids with redox-active backbones.By combining chalcogen bonding with redox chemistry, we aim to explore their use in activating bonds like C-F, C-Cl, C-S, etc., as well as in catalysis and anion-binding applications.]
Development of Base Metal Catalyst for Organoborane Synthesis
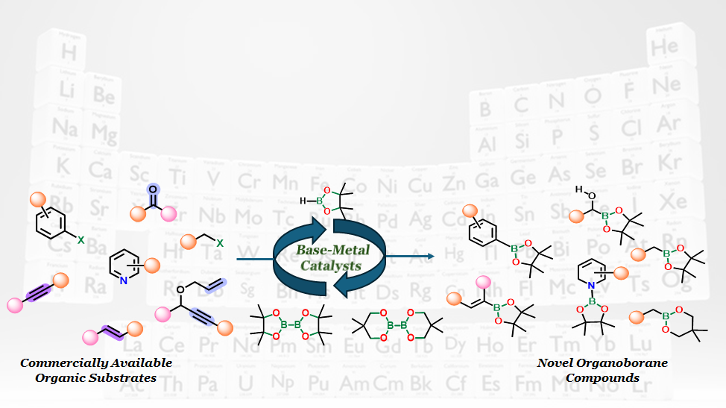
Our research group focusses on the synthesis of organoborane compounds, which are of interest to chemical, medicinal, and materials scientists because of their stability and ability to undergo selective transformations into diverse functional groups.We focus on developing new catalysts based on earth-abundant, environmentally friendly metals mostly based on Iron, Cobalt and Nickel.To effectively control the reactivity of these metals, we have designed new N-heterocyclic carbene ligands that finely tune the electronic properties of the catalytic center.This approach enables enhanced productivity and selectivity, particularly in the synthesis of organoboranes.
Photocatalysis by Main Group and Transition Metal Compounds
Light-Responsive Smart Catalysts: The Next Generation of Controllable Chemistry
Our group is working on designing and synthesising photoswitching catalysts—smart materials whose activity can be controlled using light.By responding to light, they offer more control, reduce waste, and support greener, more efficient chemistry.
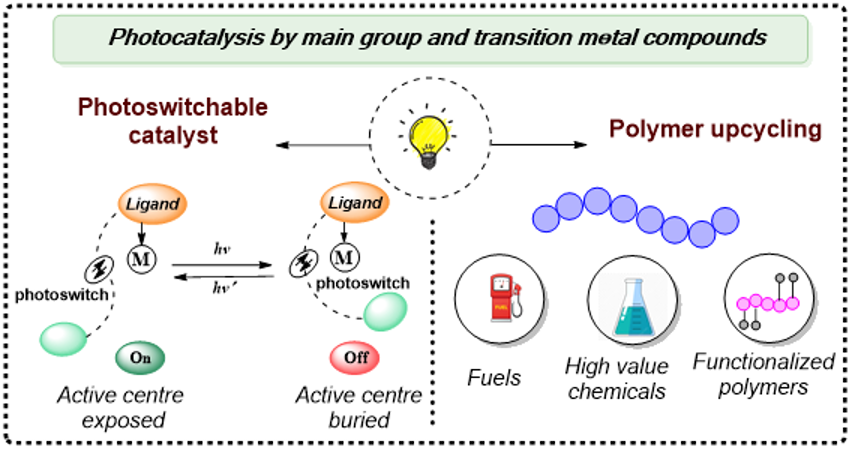
Photocatalytic upcycling of polymers using Main Group Compounds
Plastics are everywhere, but their recycling rates remain quite low, leading to significant environmental issues. Inspired by this, we are trying to effectively upcycle various polymers using main group compounds as photocatalysts, which is a better alternative compared to many prevalent harsh conditions.
Designing Novel Bioactive Boron-Containing Molecules
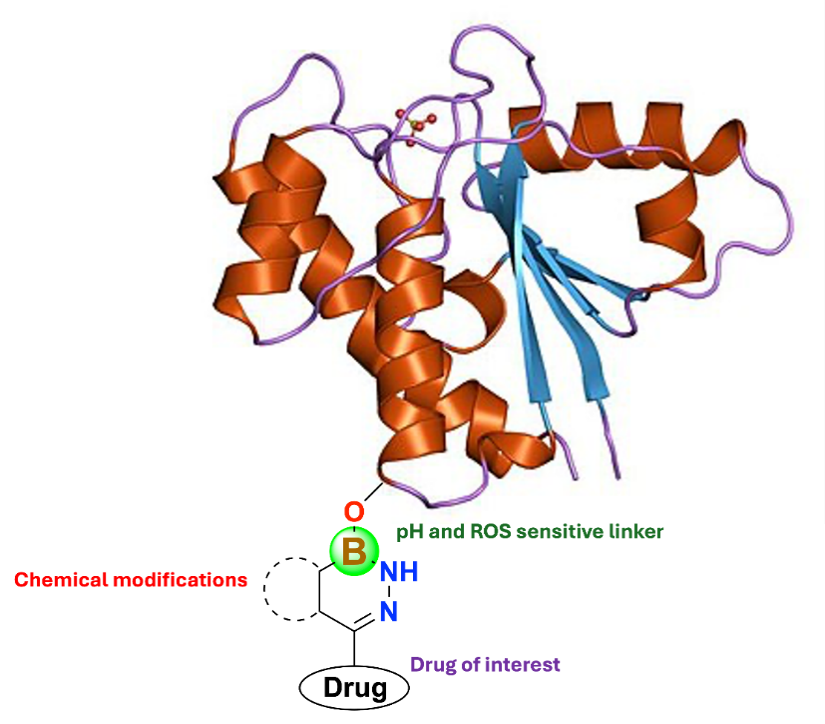
Boron’s unique electronic structure gives it mild Lewis acidity, allowing it to form covalent bonds and hydrogen bonds with biological targets in trivalent or tetracoordinated forms.This property enables the synthesis of novel bioactive molecules.Our research, in collaboration with Dr. N Ravi Sundaresan (MCBL), focuses on SIRT1-7 and PARP proteins and their influence on various systems.We also utilize molecular docking and molecular dynamics simulations to identify binding pockets and validate the affinity of potential drug candidates.
