Synthetic chemistry has grown significantly in the last few decades. Several new catalysts and technologies have opened new doorways for the rapid access to molecules of interest. New interdisciplinary fields in chemistry come into prominence with the help of novel technologies related to synthetic chemistry, polymer science, drug delivery, and nanoscience. However, these synthetic advancements in chemistry have also raised awareness of the environmental impacts of chemical processes. The Hazra group at IISc Bangalore is focused on research associated with green & sustainable chemistry. We aim to develop new technologies for valuable product synthesis, considering the green chemistry principle. The goals are i) development of recyclable amphiphilic molecules and use them as micelles; ii) get rid of toxic organic solvents for organic reactions, using minimum amounts of recyclable micelles or water as the reaction medium; (iii) earth-abundant metals catalysis, redox non innocent ligand design; (iv) merging chemo-catalysis with bio-catalysis: development of nano-machine; (v) Photo-Micellar Catalysis; (vi) Heterogenous catalysis in water with atomically dispersed catalysts.
Micellar Catalysis: The chemistry in water
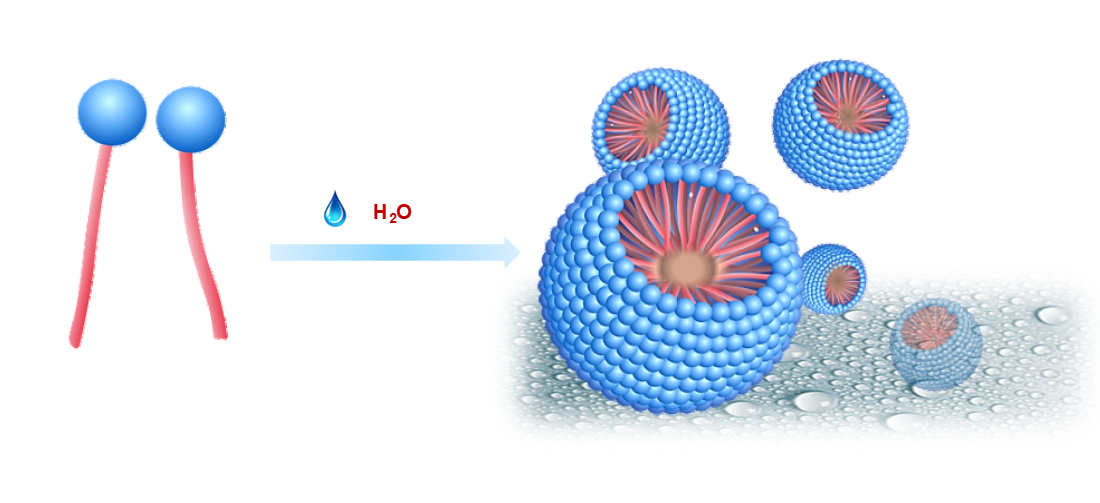
Water, the solvent for life! However, until recently, water was considered the worst enemy for synthetic chemists, and efforts had been made to avoid trace amounts of water from the reactants or organic solvents. Micellar catalysis is an enabler to perform chemistry in water under very mild conditions, thus mimicking Nature. The nano-/or microconfinement of micelles acts as a reactor to solubilize reactants and catalysts in aqueous conditions.
Bio-Micellar Catalysis
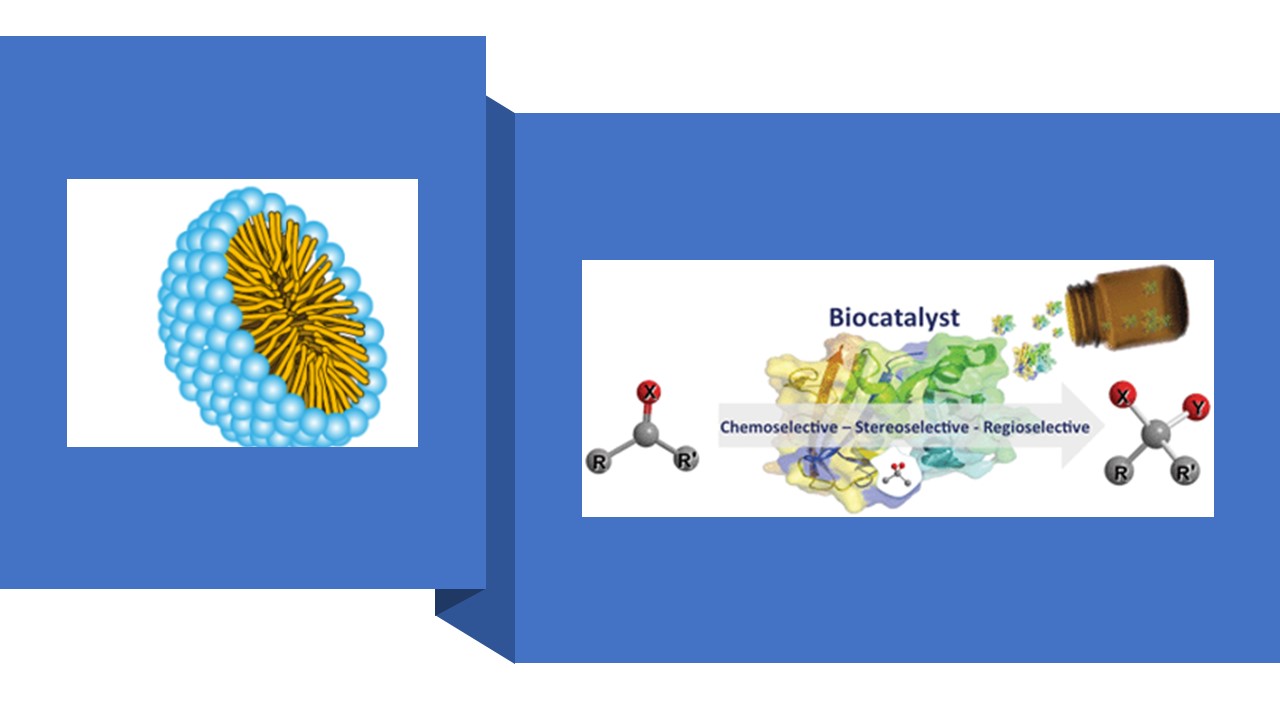
The integration of biocatalysis with chemocatalysis, which combines the environmentally friendly feature of biocatalysts with the versatile reactivity of chemical catalysts, is among the most active research areas. Inspired by Nature, the combination of biocatalysis and chemocatalysis in a cascade fashion without the need to isolate intermediates will drastically reduce the operating time, cost, and waste generation. These approaches also avoid the problems associated with the handling of unstable intermediates, thereby improving overall selectivities and yields. Additionally, the cooperative effects of multiple catalysts often lead to unique reactivity and better outcomes that cannot be achieved by the sequential application of individual catalysts. Numerous examples of combining biocatalysis and chemocatalysis in a cascade fashion have been reported. However, a major challenge is the issue of incompatibility between biocatalysts and chemocatalysts, as they are usually performed in different reaction media and conditions. Inspired by Nature, micellar catalysis was developed as an attractive alternative strategy to disperse lipophilic species in the aqueous system.
Organometallic/Nanocatalysis
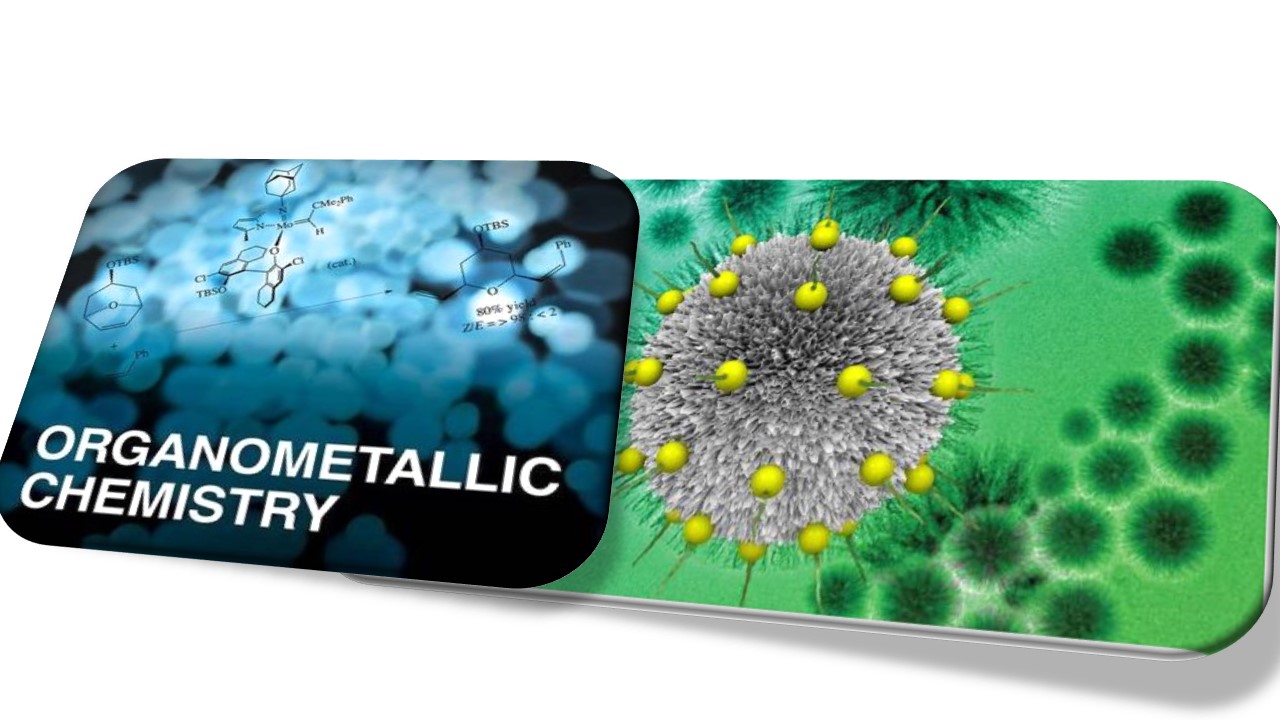
Designing a multi-functional catalyst is a challenging task because each transformation requires different, often incompatible, catalytically active sites (e.g., acidic and basic sites) and/or reaction conditions. Along this direction, the heterometallic nanoparticle (NP) catalysts have emerged as an important methodology to obtain materials with improved chemical and physical properties. The improved properties of the heterometallic nanoparticle catalysts arise due to the electronic exchange (cooperative/synergic effect) that enhances the overall reactivity and selectivity. However, most of the cases require supporting materials to prevent the agglomeration or polymerization of metal NPs, and cascade catalysis has been performed in toxic organic solvents. We are focusing on the innovation/designing surfactant that can stabilize heterometallic nanocatalysts based on earth-abundant TM. We will use these catalysts for nonconventional but greener transformations via mimicking Nature (in cascade fashion), removing toxic solvents for valuable transformations.
