
|
THEORETICAL ELECTROCHEMISTRY |

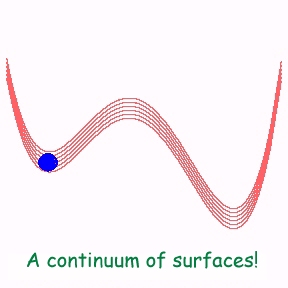
Normally, a reaction may be modelled as the escape of a particle from one side of a double well to the other side. The theory of this is well developed (known as the Kramers problem - for a generalization to the case of polymers, see the section on polymers). Now suppose the reaction takes place on a metal surface. Then, due to the existence of a continuum electronic (e-h) excitations, there are is contiuum of potential energy surfaces to be considered! See the second figure below. When the reaction takes place on such a continuua, the particle can change from one surface to the other, making the reaction non-adiabatic. An interesting question is: how to account for all these surfaces? We have used a bosonization technique to do this. The continuum of excitations is treated as a collection of bosons and then the proble can be easily handled. Simply stated, this means that each e-h excitation is replaced by a harmonic oscillator.
The method was applied to electrochemical proton transfer, where one has the possibility of proton tunneling too. It was found that the coupling to the continuum can lower the rate of the reaction by an order of magnitude.
- K. L. Sebastian: Electronic friction in proton tunneling at the electrochemical interface. J. Chem. Phys., 109, 1111 (1998).
- K. L. Sebastian: Electrochemical electron transfer: accounting for electron-hole excitations in the metal. J. Chem. Phys., 90, 4096 (1988).