
|
Molecular Devices Nanotechnology and Surface Physics |
Dynamical Effects in Scanning Tunneling Microscopy
We have investigated dynamical effects in tunneling in Scanning Tunneling Microscopy. It is well known that in the case of metals, the image interaction can lead to significant lowering of the barrier height. The image interaction arises because of the displace of surface plasmons of the metal. We ask: what happens when the tunneling time is comparable to the time required to displace the plasmons? It was found that dynamical image interaction can modify the barrier height for tunneling considerably, and that the effect was more important than believed earlier. We have also studied the barrier height for tunneling in electrochemical scanning tunneling micrscopy and found that solvent reorganization cannot happen during tunneling and hence the consequent lowering of the barrier height is negligible. (see publications 1,2 &3)
Using Fluxionality to design molecules that can move on surfaces
Fluxionality of molecules is quite well known and has been thoroughly investigated. We consider the question: can one use fluxionality to design molecules that can move on a surface? The question is of interest and importance in connection with nano-technology, where the dream is to design nano-motors that make use of chemical energy or nano-robots that can go inside a cell and perform useful work. We explore the possibility of having a molecule chemically bonded to the surface, and capable of moving on the surface because of its fluxional nature.
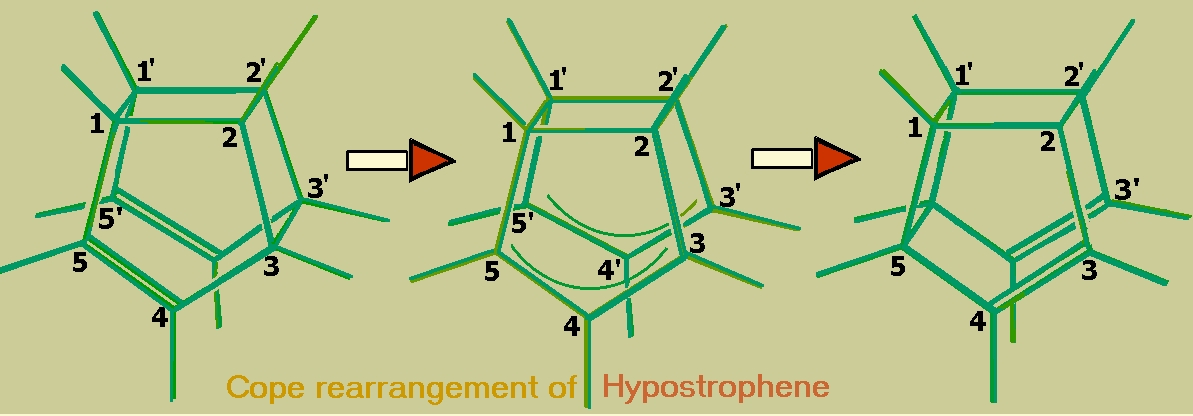
It is well known that hypostrophene (see figure) undergoes degenerate Cope rearrangement and hence is a fluxional molecule. We have performed a theoretical investigation of hypostrophene adsorbed on the (100) surface of Aluminum.
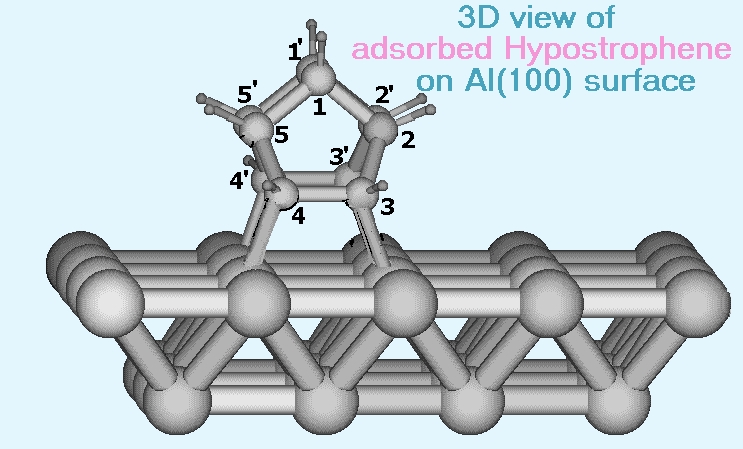
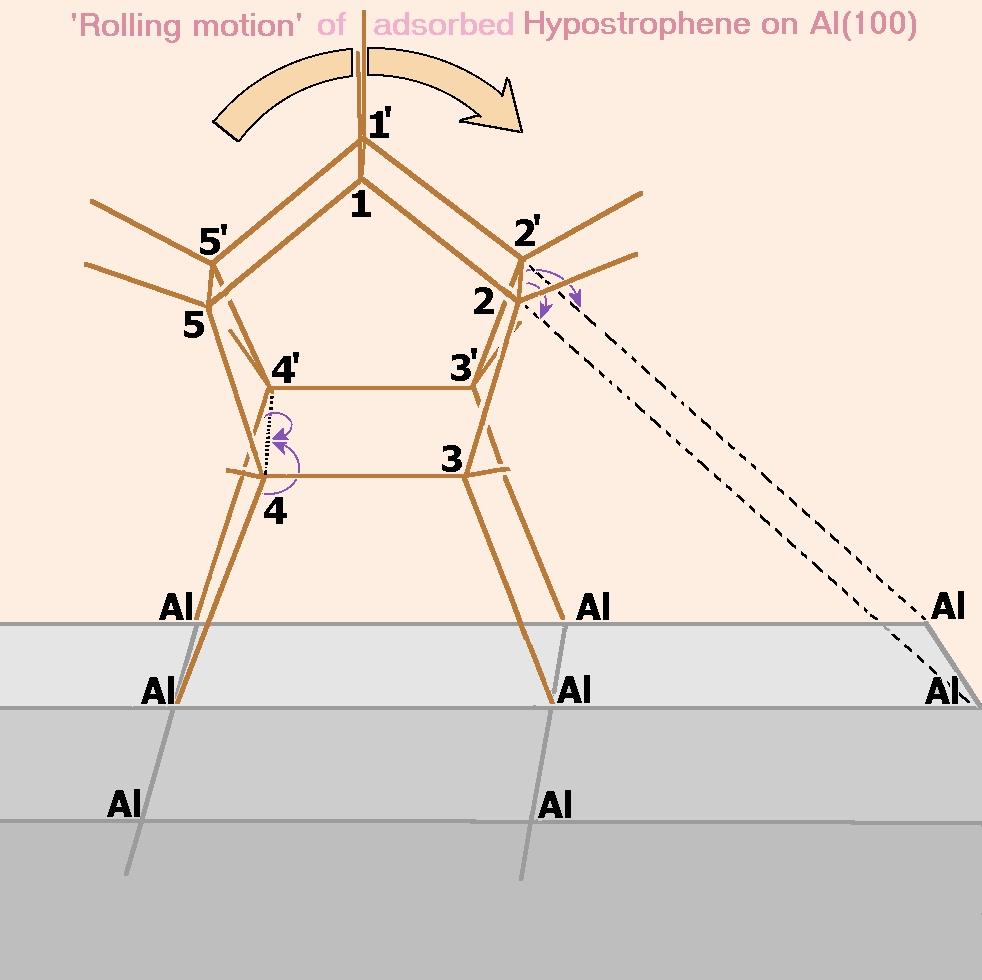
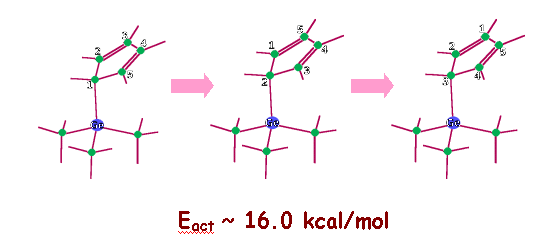
We find that chemisorbed hypostrophene can undergo a similar rearrangement involving only single bonds with an activation energy of approximately 16.9 kcal/mol. This rearrangement involves a rolling motion of the molecule on the surface and the result is a hypostrophene molecule adsorbed on the neighboring site. In comparison with this, the usual diffusional (sliding) motion has an activation energy about five times larger and hence we expect this rolling to be the dominant mechanism of diffusion of the molecule on the surface. The activation energy for degenerate Cope rearrangement of free hypostrophene is found to be 22.2 kcal/mol (theoretical) and the metal brings down this value, even though theadsorbed molecule has no double bonds in it. (see publication 4)
Molecular Wheel (Seal!)
The molecule (CH3)3Ge(C5H5) is known to
be fluxional, with an activation energy of 16 kcal/mol. See the figure for the
rearrangement that occurs.
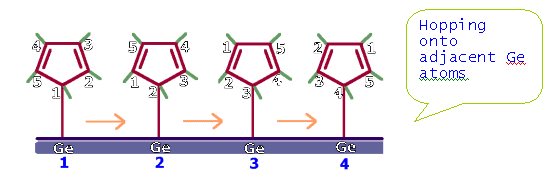
So perhaps, one can
have cyclopentadienyl adsorbed on a Ge surface and it can roll on the surface
like a wheel! (see figure 2).
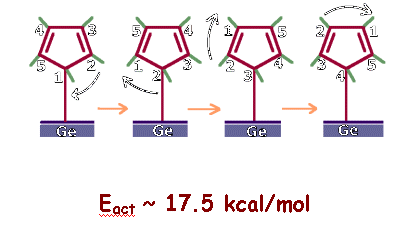
However, our
calculations lead to the result that cyclopentadienyl adsorbs on Ge surface
with three C atoms attached to different Germaniums.
Umbrella Inversion in a Molecular Rattle
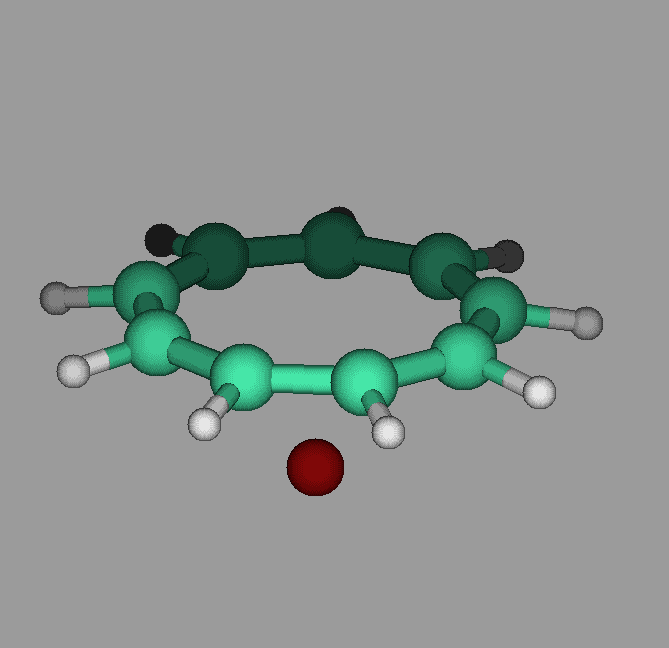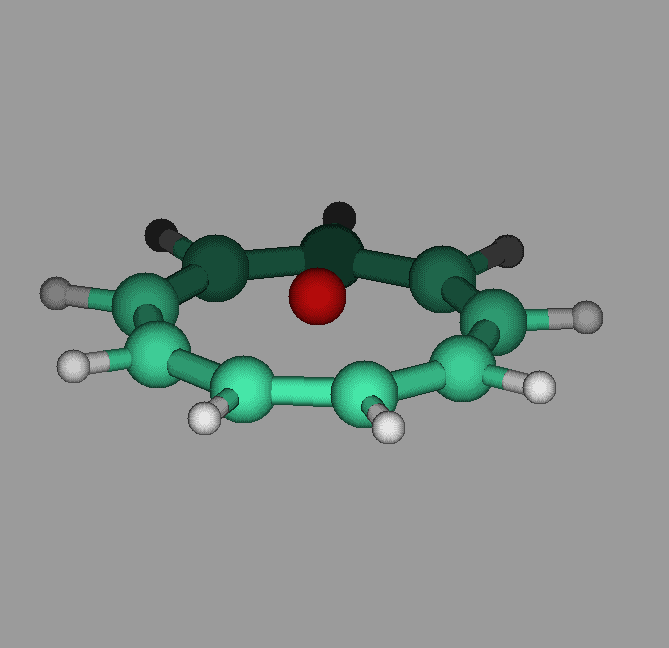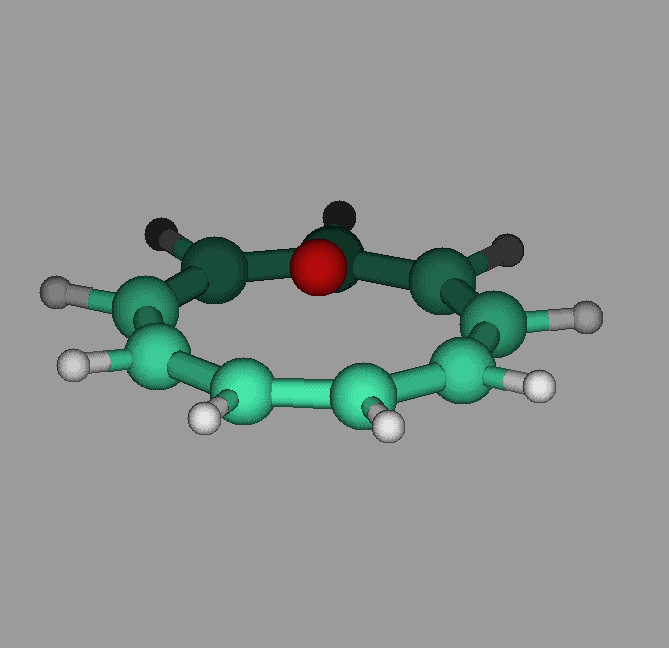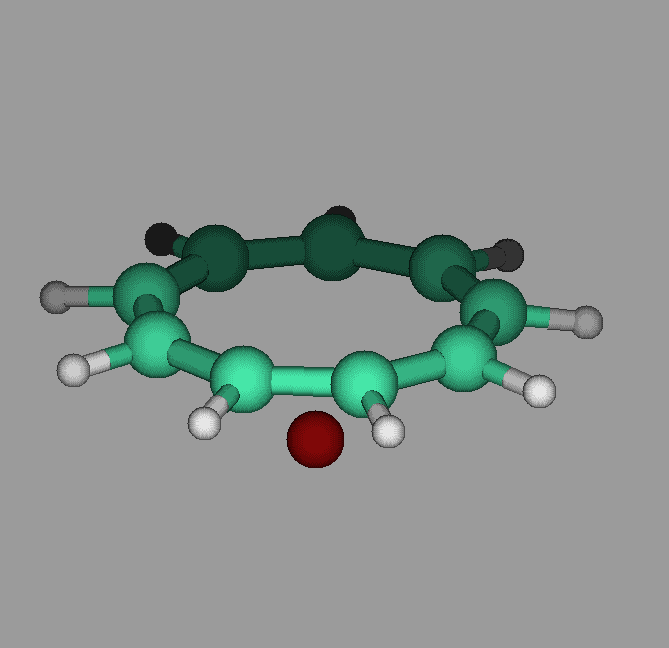
The molecule cyclononatetraenyl lithium has a pyramidal geometry. Our calculations show that it can undergo umbrella like inversions in which the lithium goes through the ring to the other side. The activation energy was found to be about 12 kcal/mol. Proper vibrational excitation would allow the ring to go from one side to the other(see figure).
- K. L. Sebastian and G. Doyen: Dynamical Image interaction in scanning tunneling microscopy. Physical Review B, 47, 7634 (1993).
- K. L. Sebastian and G. Doyen: Electrochemical scanning tunneling microscopy: does the orientational polarization of the liquid play any role?. Surface Science, 290, L703 (1993).
- K. L. Sebastian and G. Doyen: Tunneling in presence of coupling to other modes: Application to scanning tunneling microscopy. Journal of Chemical Physics, 99, 6677 (1993).
- B. Das and K. L. Sebastian: Adsorbed Hypostrophene: Can it roll on a surface by rearrangement of bonds? Chemical Physics Letters, 330, 433 (2000).
- B. Das and K. L. Sebastian: Molecular Wheels on Surfaces. Chemical Physics Letters, 351/1-2, 25 (2002).
- B. Das and K. L. Sebastian: Through Ring Umbrella Inversion of a Molecular Rattle. submitted, (2002).