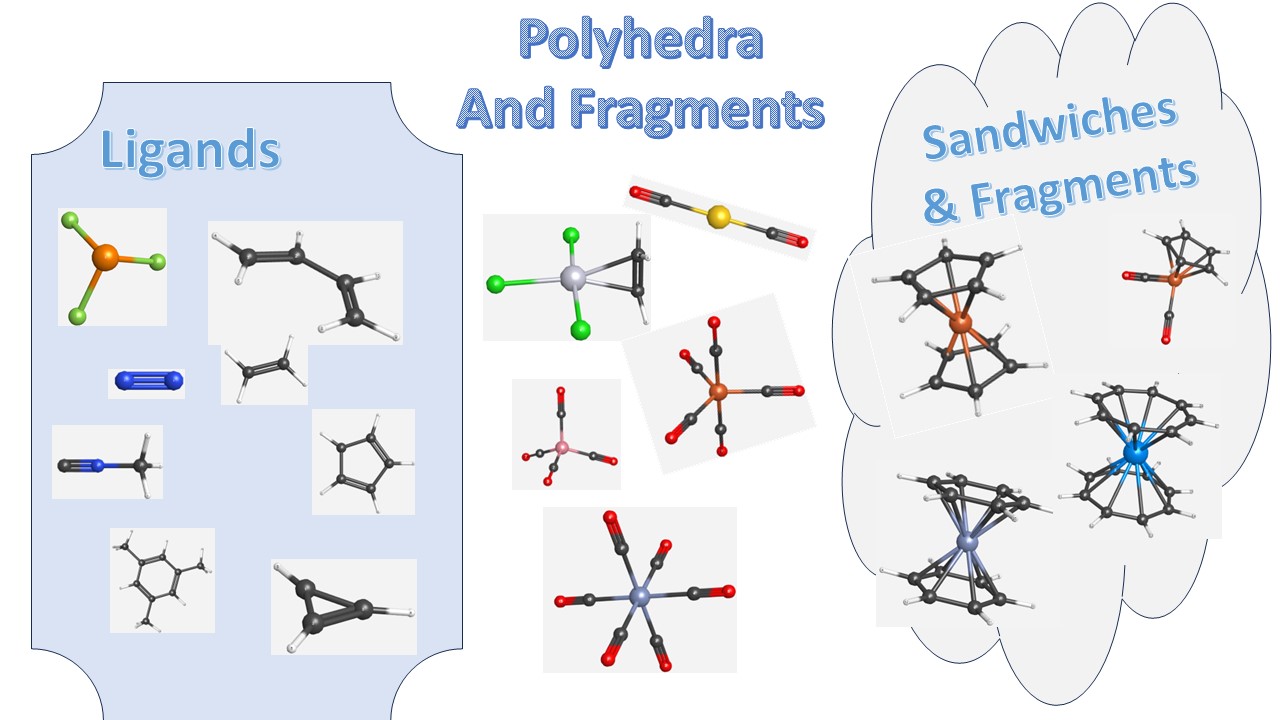
Each page gives you a group of ligands, organometallic complexes based on the closest symmetric polyhedra they resemble or Sandwich complexes and their fragments.
All web pages were generated using WEBMO and the calculations were carried out using GAUSSIAN suite of programs.
Using these web pages effectively requires some
tricks which are given here in the link: Short Video on
COMFORT (https://youtu.be/WZ3-eGsOiBs)
If
you wish to receive a of the book that accompanies the website, send a
mail to ashoka@iisc.ac.in with COMFORT
as the Subject
